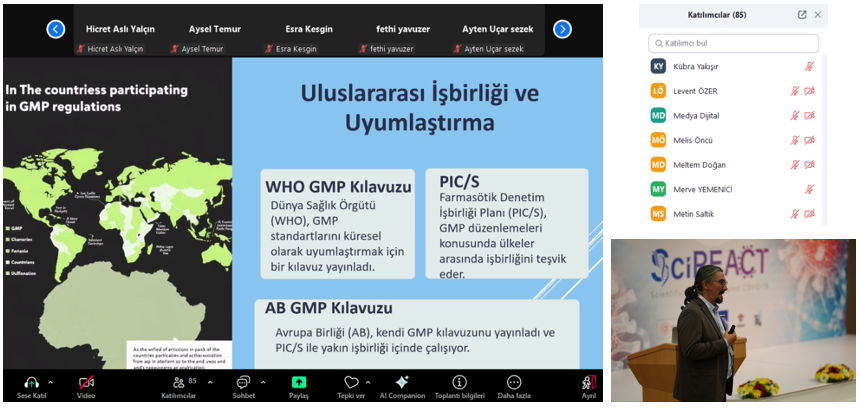
Technical requirements, quality approaches, regulations, inspection mechanisms, and sectoral experiences regarding GMP processes in biotechnological drug and vaccine production were shared.

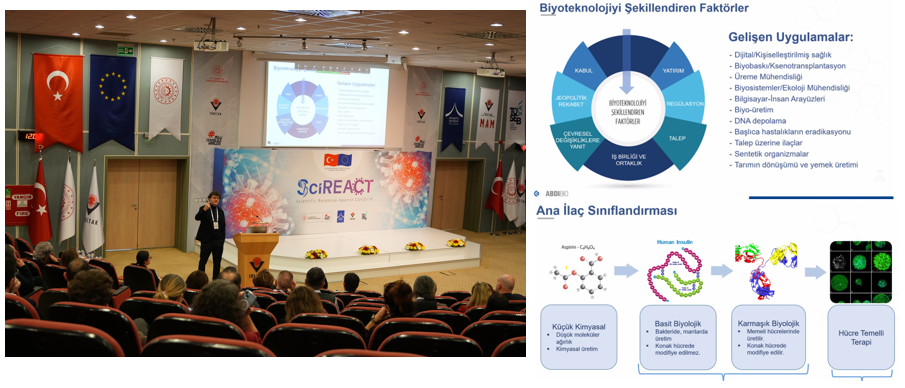
The 5th Knowledge Day of the Scientific Response Against COVID-19 (SciREACT) Project, conducted in partnership between Turkiye and the European Union, was held on January 21, 2025, at TÜBİTAK Gebze Campus UME Conference Hall in a hybrid format (physical and online). The event brought together leading industry experts and academics to share technical requirements, quality approaches, regulations, inspection mechanisms, and industry experiences in GMP processes for biotechnological drug and vaccine production.
The opening speech was delivered by Associate Professor Dr. Özgen Ercan, Acting Vice President of TÜBİTAK Marmara Research Center. Dr. Ercan emphasized the importance of GMP processes in biotechnological drug and vaccine production within the SciREACT Project supported by Turkiye and the European Union, highlighting their role in combating the pandemic and establishing sustainable quality standards in the healthcare sector.
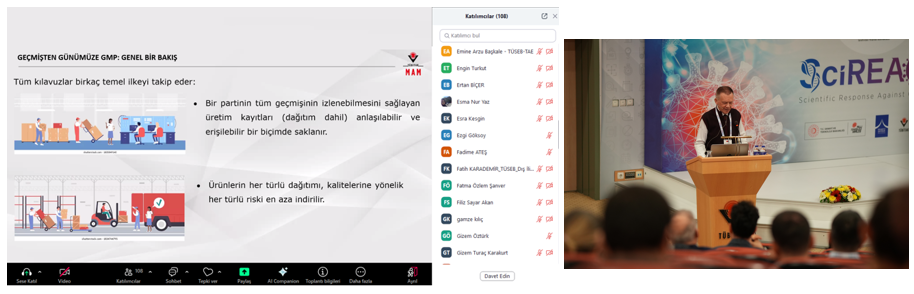
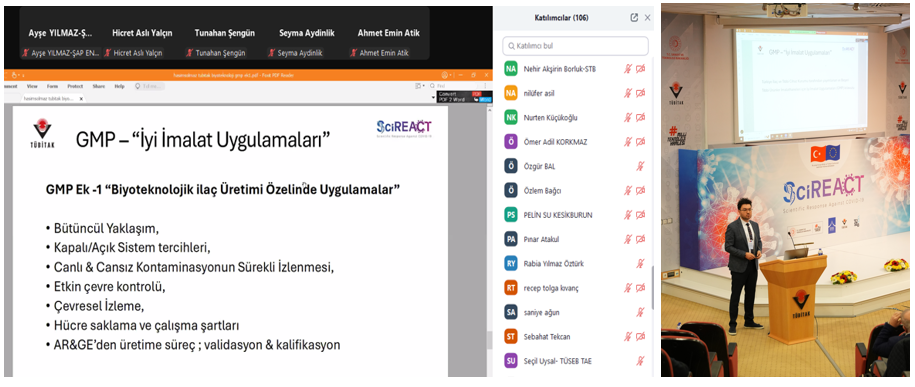
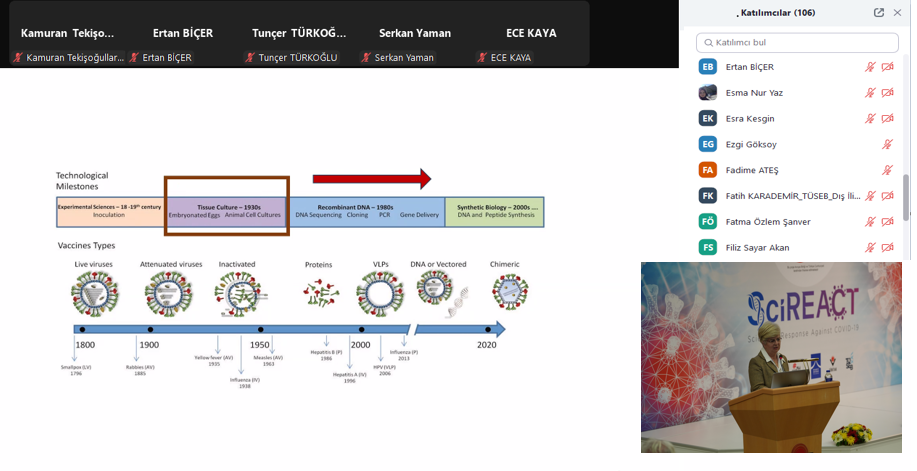
In the first session, Associate Professor Dr. Hilal Yazıcı Malkoçoğlu, TÜBİTAK MAM BYIYB Biotechnology Group Leader, discussed the importance of quality standards and GMP applications in research and solutions developed against the pandemic in her presentation "TÜBİTAK MAM's Journey in Biotechnological Drug and Vaccine Development." Following this, Dr. Recep Burak Dürüs from TÜBİTAK MAM BYIYB Biotechnology Research Group presented "GMP from Past to Present: An Overview," covering the historical development of GMP, current applications, and inspection processes in biotechnological production.
The program continued with Deniz ALKANAT, General Manager of BPEG Consulting, presenting "Technical Requirements for GMP Area Setup and Advanced Technology Processes in Biotechnological Drug and Vaccine Production Processes," discussing technical standards and advanced technology processes required for establishing GMP-compliant biotechnological production areas. Haşim SOLMAZ, General Manager of Lighthouse Worldwide Solutions EMEA Operations, addressed sterilization, particle tracking, and contamination control methods in biotechnological production in his presentation "GMP Perspective on Contamination Control in Biotechnological Drug Production."
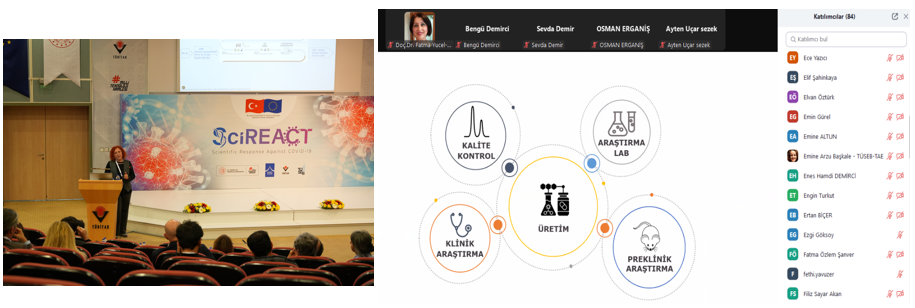
Dr. Ali ÖZÜER, AbdiBio Group President, discussed the evolution of biotechnology and AbdiBio's significant work in his presentation "Biotechnology's Past, Present, Future, and AbdiBio." Sema NOMAK, Co-founder of RS Research, addressed GMP processes from a start-up perspective. Dr. Şükran YILMAZ, Head of Cell and Virus Bank Department at Şap Institute, emphasized the importance of cell culture-based vaccine production processes and applied GMP principles.
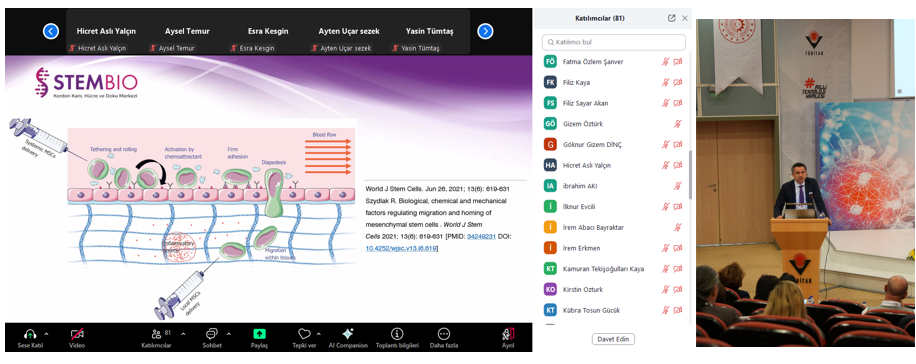
In the afternoon sessions, Dr. Nilay ÜNAL, Deputy General Manager and R&D Coordinator of Dolvet A.Ş., discussed current cGMP regulations in vaccine production and their impact on production processes. Associate Professor Dr. Durmuş BURGUCU, Medical Director of STEMBIO, evaluated the challenges in transitioning human cell and tissue products from laboratory to clinical applications and the GMP regulations applied in these processes. Ersel TAŞÇI, Education Consultant at Academia Life Sciences Center, provided important information on data security in drug production and how to protect this data in terms of risk management.
In the closing sessions, Mustafa HASAN, Founder of M2H, detailed the basic principles of pharmaceutical quality systems and GMP applications in drug production. Ceyda Akşit ŞENER discussed FDA's role and historical development in the development of biotechnological drugs in her presentation "A Brief Journey Through FDA History." In the final session, Dr. Abdullah İbrahim BÜYÜKKURT, Head of the Drug Inspection Department and Chief Inspector at TİTCK, and Dr. Çağrı İPEK, Drug Inspection Department Inspector at TİTCK, provided valuable information on the importance of GMP processes in the inspection phase and their operation in the inspection dimension.
Following the presentations, participants discussed the role of GMP processes in biotechnological drug and vaccine production, basic principles of pharmaceutical quality systems, importance of GMP applications in drug production, regulations and inspection mechanisms, and how production processes can be effectively managed. Finally, participants had the opportunity to explore more closely the contributions of GMP applications in biotechnological drug and vaccine production to healthcare technologies and product development processes during their visit to TÜBİTAK MAM Vaccine and Drug Development Campus.